Evaluation of ozonated oil and centella asiatica extract on excisional wound healing in Mice
Abstract
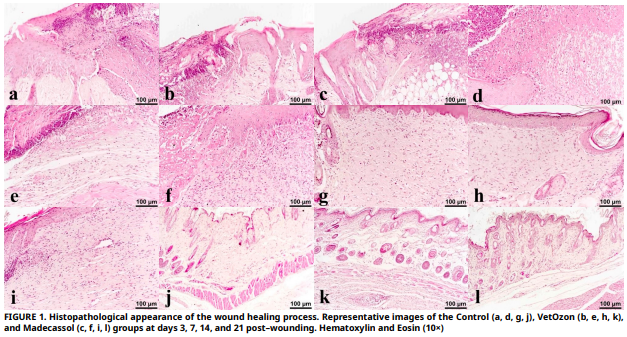
The aim of this study was to evaluate the effects of two topical treatments on wound healing in BALB/c mice: ozonated oil and an Centella asiatica extract. On days 3, 7, 14, and 21, the inflammatory response, re–epithelialization, collagen deposition, and the expression of growth factors such as Vascular Endothelial Growth Factor, Fibroblast Growth Factor, and Transforming Growth Factor– beta were assessed in order to evaluate healing. When compared to the control, VetOzon and Madecassol both markedly elevated Transforming Growth Factor–beta and Vascular Endothelial Growth Factor expression in the first phase. By day 7, Madecassol encouraged more advanced re–epithelialization and fibrosis, while VetOzon produced a more marked inflammatory response, which was necessary to start the healing cascade. The superiority of both treatments was evident by day 14, where they achieved complete re–epithelialization, a milestone the control group had not reached. Notably, Madecassol demonstrated significantly higher collagen deposition than both VetOzon and the control on days 14 and 21, indicating superior extracellular matrix synthesis. By day 21, wounds treated with either agent showed advanced remodeling with reduced cellularity and hair follicle regeneration, while control wounds appeared stalled in an earlier healing phase. In conclusion, both agents effectively accelerate cutaneous wound healing, but through complementary pathways. VetOzon acts as an early–phase inflammatory catalyst, making it potentially suitable for chronic or infected wounds, whereas Madecassol functions as a sustained anabolic stimulator, positioning it as an ideal agent for promoting high–quality, regenerative healing in clean wounds.
Downloads
References
Reinke JM, Sorg H. Wound repair and regeneration. Eur. Surg. Res. [Internet]. 2012; 49(1):35–43. doi: https://doi.org/f3462m DOI: https://doi.org/10.1159/000339613
Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: An update from physiopathology to current therapies. Life [Internet]. 2021; 11(7):665. doi: https://doi.org/gpzjr3 DOI: https://doi.org/10.3390/life11070665
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. [Internet]. 2017; 58(1–2):81–94. doi: https://doi.org/gh2dqr DOI: https://doi.org/10.1159/000454919
Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. [Internet]. 2024; 25(8):599–616. doi: https://doi.org/gt25gn DOI: https://doi.org/10.1038/s41580-024-00715-1
Akita S. Wound repair and regeneration: Mechanisms, Signaling. Int. J. Mol. Sci. [Internet]. 2019; 20(24):6328. doi: https://doi.org/g7j69v DOI: https://doi.org/10.3390/ijms20246328
Zhang Y, Lu Q. Immune cells in skin inflammation, wound healing, and skin cancer. J. Leukoc. Biol. [Internet]. 2024; 115(5):852–865. doi: https://doi.org/qmg2 DOI: https://doi.org/10.1093/jleuko/qiad107
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: A cellular perspective. Physiol. Rev. [Internet]. 2019; 99(1):665–706. doi: https://doi.org/ghzzr5 DOI: https://doi.org/10.1152/physrev.00067.2017
Tyavambiza C, Meyer M, Meyer S. Cellular and molecular events of wound healing and the potential of silver based nanoformulations as wound healing agents. Bioeng. [Internet]. 2022; 9(11):712. doi: https://doi.org/qmg4 DOI: https://doi.org/10.3390/bioengineering9110712
Yuliati L, Mardliyati E, Bramono K, Freisleben HJ. Asiaticoside induces cell proliferation and collagen synthesis in human dermal fibroblasts. Universa Med. [Internet]. 2015; 34(2):96–103. doi: https://doi.org/qgts DOI: https://doi.org/10.18051/UnivMed.2015.v34.96-103
Sun B, Wu L, Wu Y, Zhang C, Qin L, Hayashi M, Kudo M, Gao, M, Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: a review. Front. Pharmacol. [Internet]. 2020; 11:568032. doi: https://doi.org/gpswgv DOI: https://doi.org/10.3389/fphar.2020.568032
Anzolin A, Da Silveira–Kaross N, Bertol C. Ozonated oil in wound healing: what has already been proven? Med. Gas Res. [Internet]. 2020; 10(1):54–59. doi: https://doi.org/gp7jx5 DOI: https://doi.org/10.4103/2045-9912.279985
Brito Júnior AAD, Carneiro JKMP, Reis JVNA, Oliveira TJS, Dantas JBDL. Application of ozonized oils in human body and oral cavity systems. RGO – Rev. Gaúch. Odontol. [Internet]. 2022; 70:e20220027. doi: https://doi.org/qmg5 DOI: https://doi.org/10.1590/1981-86372022002720200152
Ugazio E, Tullio V, Binello A, Tagliapietra S, Dosio F. Ozonated oils as antimicrobial systems in topical applications. their characterization, current applications, and advances in ımproved delivery techniques. Molecules [Internet]. 2020; 25(2):334. doi: https://doi.org/gkcf6q DOI: https://doi.org/10.3390/molecules25020334
Cho K–H, Kim J–E, Bahuguna A, Kang D–J. Ozonated sunflower oil exerted potent anti–ınflammatory activities with enhanced wound healing and tissue regeneration abilities against acute toxicity of carboxymethyllysine in Zebrafish with ımproved blood lipid profile. Antioxidants [Internet]. 2023; 12(8):1625. doi: https://doi.org/qmg6 DOI: https://doi.org/10.3390/antiox12081625
Lim Y, Lee H, Woodby B, Valacchi G. Ozonated oils and cutaneous wound healing. Curr. Pharm. Des. [Internet]. 2019; 25(20):2264–2278. doi: https://doi.org/g9mvfw DOI: https://doi.org/10.2174/1381612825666190702100504
Ruiz–Vall A, Altamirano–Faus A, Bedmar–Gonzalez N, Andres– Lencina JJ. Improvement of surgical wound healing with ozonated oil in bilateral breast surgery: A pilot ıntra–patient comparative study. Cureus [Internet]. 2025; 17(6):e85829. doi: https://doi.org/qmg7 DOI: https://doi.org/10.7759/cureus.85829
Xiao W, Tang H, Wu M, Liao Y, Li K, Li L, Xu X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. [Internet]. 2017; 37(6):BSR20170658. doi: https://doi.org/fz27 DOI: https://doi.org/10.1042/BSR20170658
Ginel PJ, Negrini J, Guerra R, Lucena R, Ruiz–Campillo MT, Mozos E. Effect of topical ozonated sunflower oil on second intention wound healing in turtles: a randomised experimental study. J. Vet. Sci. [Internet]. 2021; 22(2):e27. doi: https://doi.org/qmg8 DOI: https://doi.org/10.4142/jvs.2021.22.e27
Van De Vyver M, Boodhoo K, Frazier T, Hamel K, Kopcewicz M, Levi B, Maartens M, Machcinska S, Nunez J, Pagani C, Rogers E, Walendzik K, Wisniewska J, Gawronska–Kozak B, Gimble JM. Histology scoring system for murine cutaneous wounds. Stem Cells Dev. [Internet]. 2021; 30(23):1141–1152. doi: https://doi.org/gqp5b6 DOI: https://doi.org/10.1089/scd.2021.0124
Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. [Internet]. 2020; 10(9):200223. doi: https://doi.org/gpqrtv DOI: https://doi.org/10.1098/rsob.200223
Togi S, Togi M, Nagashima S, Kitai Y, Muromoto R, Kashiwakura J, Miura T, Matsuda T. Implication of NF–κB activation on ozone–ınduced HO–1 activation. BPB Rep. [Internet]. 2021; 4(2):59–63. doi: https://doi.org/qmg9 DOI: https://doi.org/10.1248/bpbreports.4.2_59
Gunawan ES, Budiono BP. Enhancing the early inflammatory response: The role of ozonated Aloe vera oil on IL–6 and TNF–α in cutaneous wound repair. Biosci. Med. J. Biomed. Transl. Res. [Internet]. 2025; 9(9):2852–2865. doi: https://doi.org/qmhb DOI: https://doi.org/10.37275/bsm.v9i9.1380
Ozdemir O, Ozkan K, Hatipoglu F, Uyaroglu A, Arican M. Effect of asiaticoside, collagenase, and alpha–chymotrypsin on wound healing in rabbits. Wounds [Internet]. 2016 [cited Aug 20, 2025]; 28(8):279–286. Available in: https://goo.su/xq9Y
Mamun AA, Shao C, Geng P, Wang S, Xiao J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. [Internet]. 2024; 15:1395479. doi: https://doi.org/g8qdxd DOI: https://doi.org/10.3389/fimmu.2024.1395479
Singh D, Rai V, Agrawal DK. Regulation of collagen I and collagen III in tissue injury and regeneration. Cardiol. Cardiovasc. Med. [Internet], 2023; 7(1):5–16. doi: https://doi.org/g86n33
Budi EH, Schaub JR, Decaris M, Turner S, Derynck R. TGF–β as a driver of fibrosis: physiological roles and therapeutic opportunities. J. Pathol. [Internet]. 2021; 254(4):358–373. doi: https://doi.org/gphpf8 DOI: https://doi.org/10.1002/path.5680
Shi Z, Yao C, Shui Y, Li S, Yan H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol, 2023; 14:1284981. doi: https://doi.org/qmhc DOI: https://doi.org/10.3389/fphys.2023.1284981